COSENTYX 150 MG INJEKČNÝ ROZTOK V NAPLNENEJ INJEKČNEJ STRIEKAČKE sol inj 1x1 ml/150 mg (striek.inj.skl.) - Príbalový leták
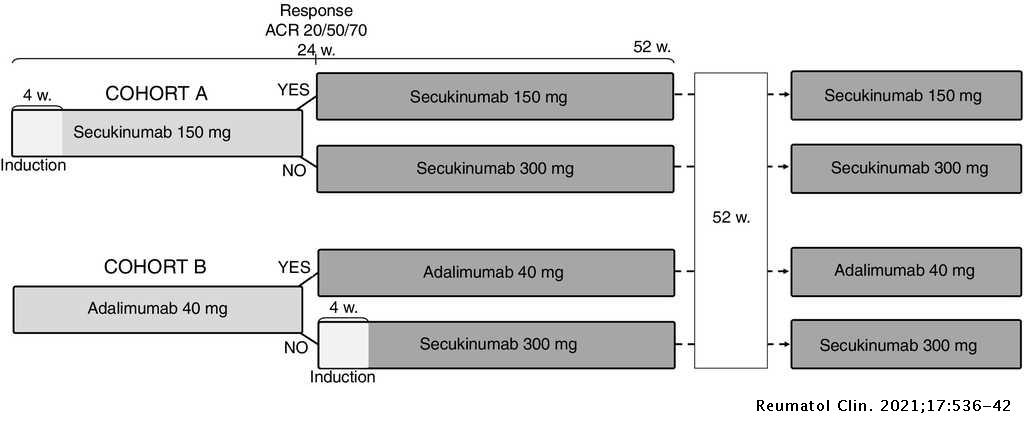
A cost-consequence analysis of the preferential use of secukinumab versus adalimumab for the treatment of psoriatic arthritis | Reumatología Clínica
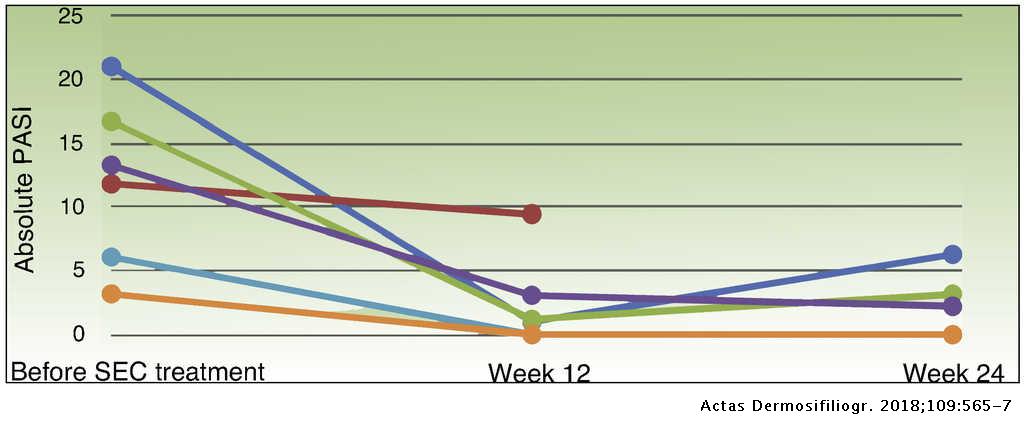
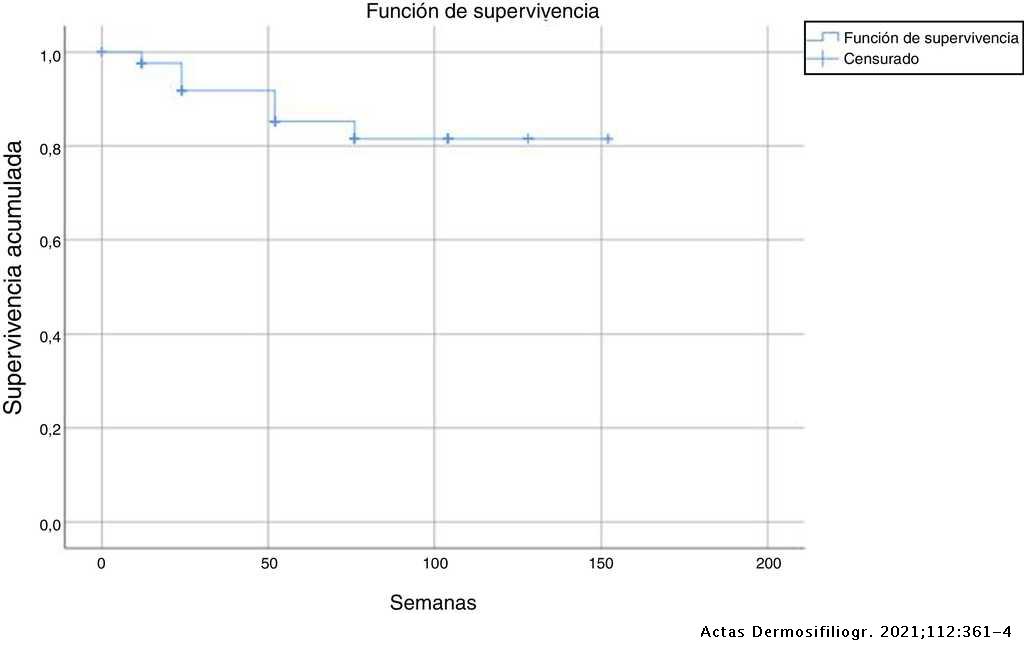
Response to Secukinumab after Treatment Failure with Ustekinumab in 6 Patients with Plaque Psoriasis | Actas Dermo-Sifiliográficas

Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect
COSENTYX 150 MG INJEKČNÝ ROZTOK V NAPLNENEJ INJEKČNEJ STRIEKAČKE sol inj 6(3x2)x1 ml/150 mg (striek.inj.skl.) - Príbalový leták

PDF) Repurposing of Secukinumab as Neuroprotective in Cuprizone-Induced Multiple Sclerosis Experimental Model via Inhibition of Oxidative, Inflammatory, and Neurodegenerative Signaling

Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect
COSENTYX 150 MG INJEKČNÝ ROZTOK V NAPLNENEJ INJEKČNEJ STRIEKAČKE sol inj 1x1 ml/150 mg (striek.inj.skl.) - Príbalový leták
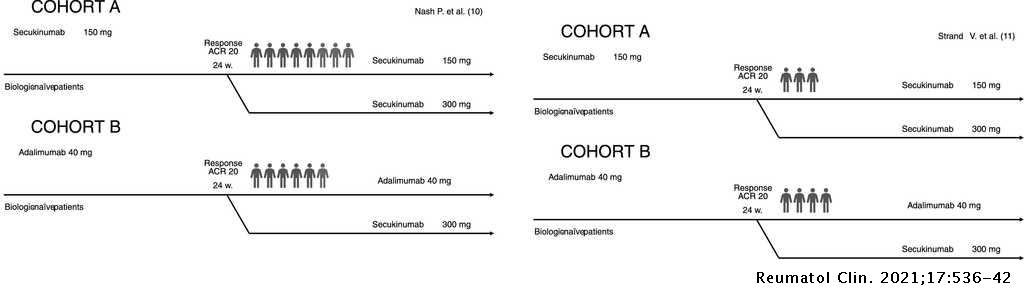
A cost-consequence analysis of the preferential use of secukinumab versus adalimumab for the treatment of psoriatic arthritis | Reumatología Clínica

Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect

Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect
Cosentyx® Novartis Biociências SA Solução injetável 150 mg/mL Contém: 1 ou 2 canetas preenchidas Bula do Paciente

Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect