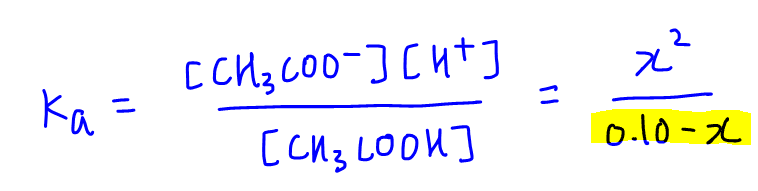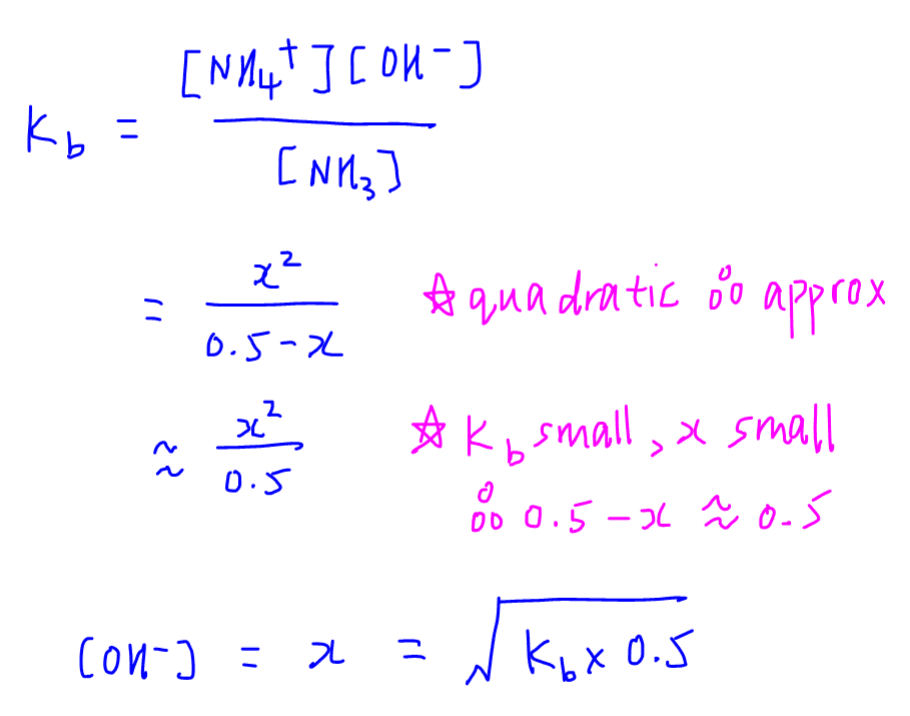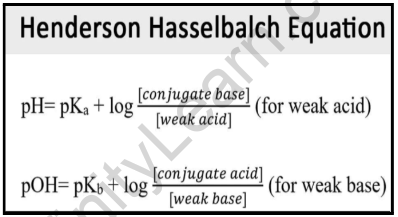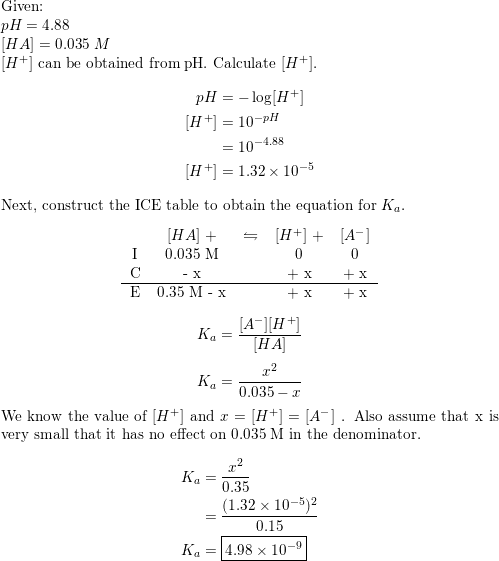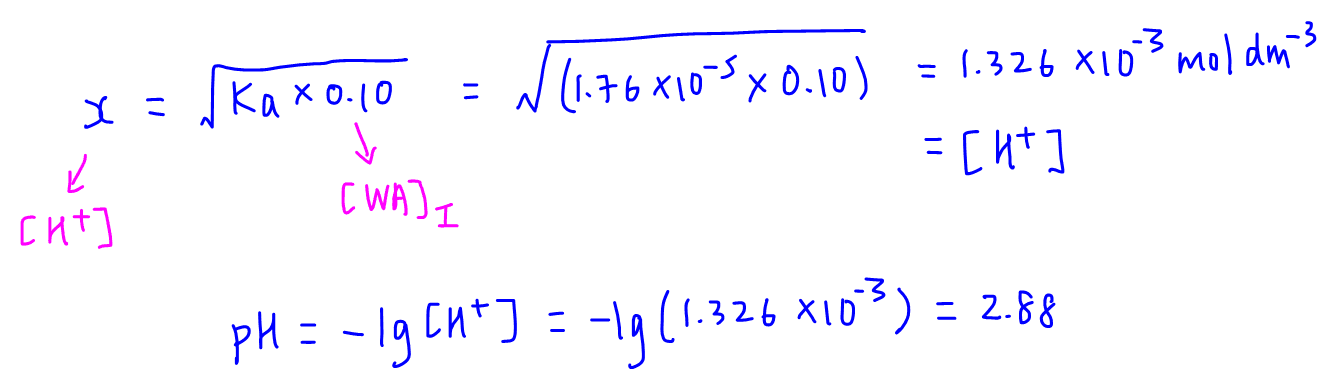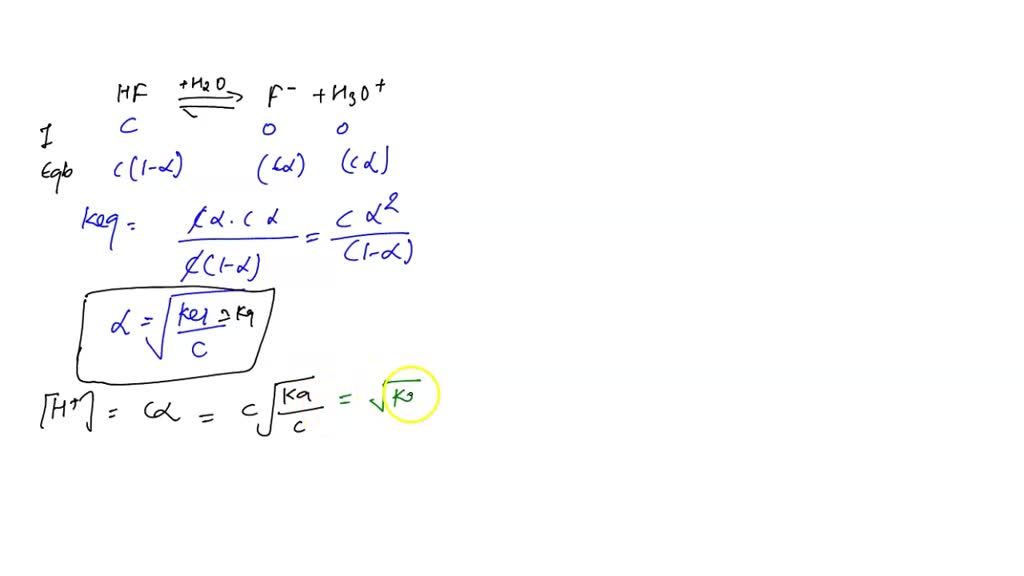
SOLVED: Calculate the pH of the weak acid HF at equilibrium, if the initial concentration of HF was 0.0340 M. (Ka = 1.45 x 10-5)
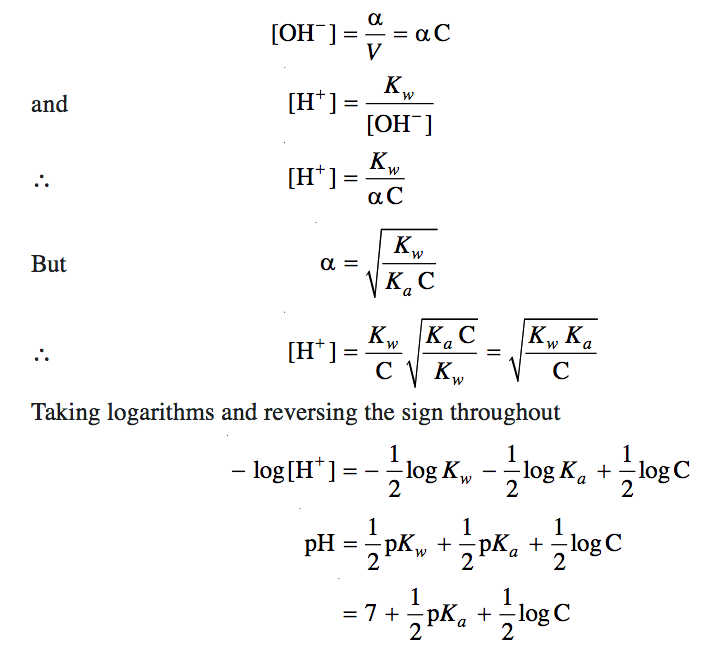
Calculation of Hydrolysis Constant, Degree of Hydrolysis and pH of Salt Solution - Chemistry, Class 11, Ionic Equilibrium