![DBU-Mediated Domino Annulation Reaction of 2-Amino-4H-chromen-4-ones and Aromatic Aldehydes for Synthesis of Polysubstituted 3-Hydroxy-5H-chromeno[2, 3-b]pyridinones | The Journal of Organic Chemistry DBU-Mediated Domino Annulation Reaction of 2-Amino-4H-chromen-4-ones and Aromatic Aldehydes for Synthesis of Polysubstituted 3-Hydroxy-5H-chromeno[2, 3-b]pyridinones | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/acs.joc.2c01152/asset/images/medium/jo2c01152_0002.gif)
DBU-Mediated Domino Annulation Reaction of 2-Amino-4H-chromen-4-ones and Aromatic Aldehydes for Synthesis of Polysubstituted 3-Hydroxy-5H-chromeno[2, 3-b]pyridinones | The Journal of Organic Chemistry

Efficient Cu(OTf)2-catalyzed and microwave-assisted rapid synthesis of 3,4-fused chromenopyridinones under neat conditions - ScienceDirect
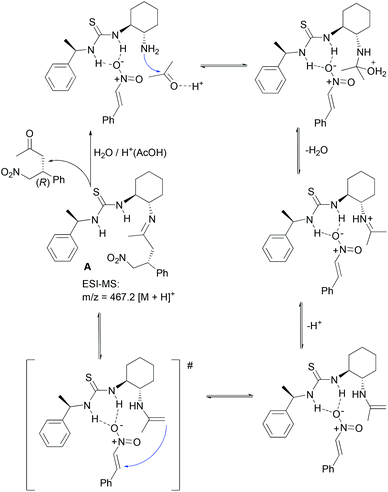
Bifunctional primary amine-thioureas in asymmetric organocatalysis - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C3OB41403E

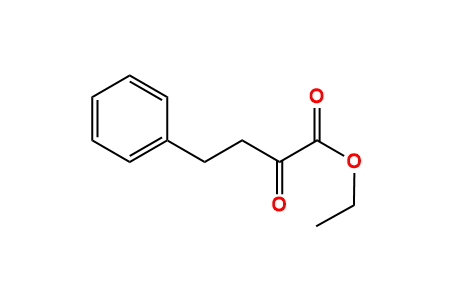
ethyl 3-oxo-4-phenylbutanoate - 718-08-1, C12H14O3, density, melting point, boiling point, structural formula, synthesis